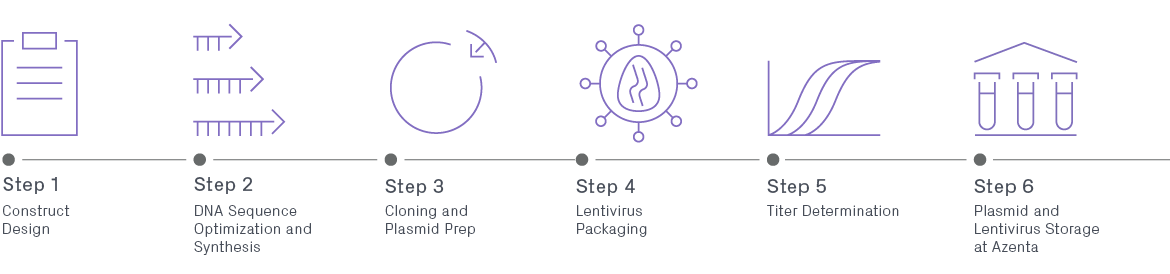
Lentivirus Production
Lentivirus Production services provide a comprehensive set of high-titer and high-throughput lentiviral solutions, spanning construct synthesis through viral production plus storage of transduction-ready virus. Lentiviral vectors are broadly used in laboratories across academia and industry for research and clinical gene therapy applications. Gene therapy vectors derived from lentivirus offer an array of unique advantages over traditional retroviral gene delivery systems. Leading these is their ability to provide long-term and stable gene expression and robust infection of both dividing and non-dividing cells, such as neurons.
With GENEWIZ Multiomics and Synthesis Solutions from Azenta Life Sciences, you can enjoy the convenience of an end-to-end solution to advance your research.
Applications of Lentivirus Vectors
What Are the Advantages of Lentivirus?
Lentiviral vectors are research tools used to introduce gene products into in vitro systems, animal models, or human patients via cell therapy. Lentiviruses have a broad range of applications and are commonly used in clinical cell therapy as well as in CAR-T cell engineering.
- Lentiviral vectors can carry relatively large transgenes
- Low immunogenicity
- Can be used for gene editing (e.g. deliver CRISPR elements to the cell)
- Ability to transduce both dividing and non-dividing cells
- Integration into the host genome and stable expression of the transgene
For more lentivirus frequently asked questions (FAQs) please visit our lentivirus FAQ page.
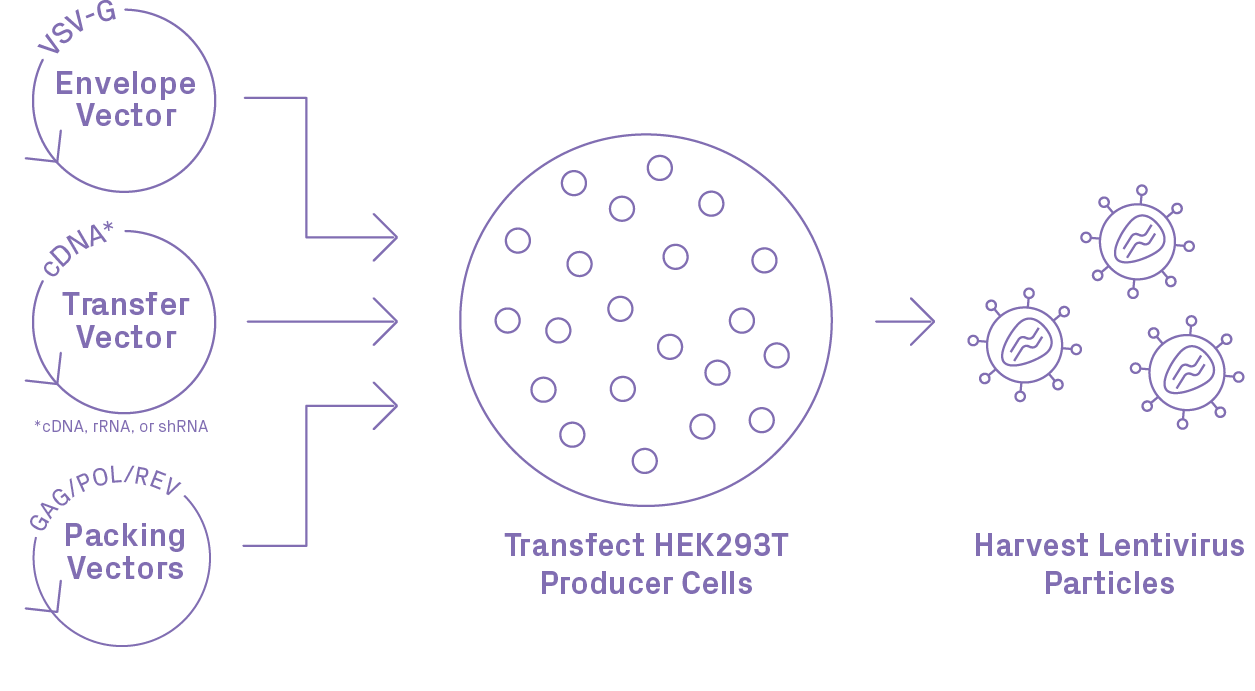
Lentiviral Packaging Workflow
GENEWIZ Lentiviral Packaging Advantages
- Convenience - One-stop shop from gene synthesis or plasmid prep to lentivirus packaging
- Flexible Synthesis Options - A variety of viral prep scales with high-titer transduction-ready virus available (>65% of all packaging achieved ~3-fold higher than the quoted amount)
- Fit-for-Screening Deliverables - High-throughput lentiviral workflows accelerate your downstream screening applications (CRISPR/Cas9, shRNA, etc.)
- Rigorous Quality Control - Functional titering option available
- Dedicated Ph.D.-Level Support – Support at every step with real-time project updates through our online system
- Scalable Project Design – Take your previously synthesized lentiviral plasmids directly to prep and packaging
Lentivirus Production Offerings
Lentivirus Production
| Virus Titer | Volume Available | Estimated Completion Time* | Quality Control |
| >108 IFU/mL | 250µL - 500µL, 1mL - 1.5mL | In as few as 2 weeks (~90% of the projects are delivered ahead of the projected TAT) | Default titration by p24 ELISA, functional titering by qPCR available upon request |
| >109 IFU/mL | 250µL - 500µL, 1mL - 1.5mL |
*Starting with ready-to-transfect transfer plasmid DNA at the recommended amount
High-Throughput Lentivirus Production
| Workflow | Pooled Format | Arrayed Format |
| Plasmid Generation | Oligo pool synthesis and high-throughput cloning into lentiviral plasmid of your choice, constructing a plasmid library | Standard gene synthesis/cloning to generate individual clonal plasmid constructs which go through the same stringent QC process |
| Plasmid Preparation | Transfection-grade plasmid DNA preparation of either clonal or library plasmids before lentivirus production | |
| Lentivirus Packaging | LV packaging of plasmid library, delivering LV particles at either 108 IFU/ml or 109 IFU/ml titers with various volume options (250ul, 500ul, 1ml, 1.5ml, etc.) | High-throughput packaging of a large quantity of arrayed plasmids, delivering LV particles in 96-well plates with 50-100ul volume per well |
Third Generation* Lentiviral Vectors
| Component | Options |
| Promoter | CMV, EF1α, U6, etc. |
| Selection Marker | Puromycin, Hygromycin, Neomycin, etc. |
| Fluorescent Marker | GFP, etc. |
| Custom Cargo Design | De novo synthesis of the entire LTR to LTR cassette to meet all requirements |
*Third generation is our default lentivirus packaging workflow and, if required, second generation can be accommodated upon review
SECOND VS THIRD GENERATION LENTIVIRUS SYSTEMS
| Component | Second Generation | Third Generation |
| Transfer Plasmid | Can be packaged ONLY by a second-generation packaging system | Can be packaged by both second and third generation packaging systems |
| Packaging Plasmid | Plasmids includes Gag, Pol, Rev, Tat for entry and integration of the viral genome | Plasmids include only packaging genes Gag and Pol |
| Envelope Plasmid | Contains gene encoding for envelope proteins | Contains gene encoding for envelope proteins |
| Safety | Safe. Replication incompetent: uses 3 separate plasmids encoding various HIV genes | Safer. Transfer plasmids are all replication incompetent rendering the virus “self-inactivating” (SIN) after integration. |
| Titer | Standard to high viral yield | Higher viral yield |
Technical Resources

Tech Note | Enhancing Protein Expression by Leveraging Codon Optimization
Codon preference is among the most critical parameters affecting the expression level of foreign genes. This technical note will discuss two practical case studies, comparing wild-type gene performance to Azenta codon-optimized sequences.

Infographic | Lentiviral Vector Packaging for Enhanced Downstream Research
The vectors derived from lentivirus offer an array of unique advantages over traditional retroviral gene delivery systems, but the process can be tedious and complex. Learn more about the critical steps and factors to consider when producing lentivirus for enhancing your downstream research.

Blog | Lentivirus Production for Gene Delivery
Lentiviruses are one of the most utilized viral delivery systems for cell and gene therapy because they have broad host-cell tropism and offer stable integration into the host cell. Here, we discuss how lentiviruses have been engineered to deliver transgenes safely and how researchers today generate lentiviral preparations.

Poster | Optimizing mRNA Vaccines and Virus-Mediated CAR T-Cell Therapy for Immuno-Oncology
Lentiviral vectors have proven effective at delivery of genetic material or gene editing technology for ex vivo processing, but the benefits and promise of Adeno-associated virus (AAV) and mRNA tools for in vivo immunotherapy have garnered recent interest. In this poster, we present streamlined approaches for lentiviral, AAV, and mRNA production that improves data quality through innovative synthesis and sequencing methodologies as compared to current standards.

 Europe (English)
Europe (English)