Integration Site Analysis for Cell and Gene Therapy
A critical portion of every cell or gene therapy study is the long-term follow-up (LTFU) observations to monitor the safety of therapeutic products. Regulatory guidance for LTFU focuses on integration site analysis (ISA) as a method to identify the location and frequency of vector integration. GENEWIZ partners with you by implementing our pre-optimized ISA protocols through pre-clinical and clinical sample collection and management over the course of your trial in our labs designed to follow Good Clinical Practices (GCP) guidelines and GLP compliance. The result is total confidence in your study data working with a single, experienced clinical trial partner.
What is Integration Site Analysis?
Integration site analysis (ISA) allows for the interrogation of a host genome for lentiviral vector insertion location and frequency after delivery of the therapeutic product. ISA is an important safety tool used to assess for clonal expansion in treated subjects.
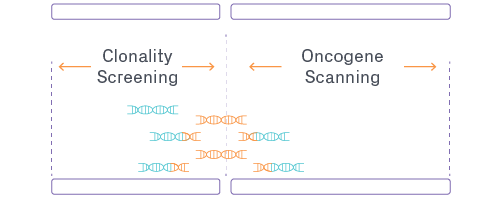
GENEWIZ Lentiviral Integration Site Analysis Workflow

Featured Application: Integration Site Analysis for Lentiviral Therapies
As the dominant viral delivery vector for approved cell therapies, lentivirus requires ISA in pre-clinical and clinical trials due to its possibility of altering expression of cellular genes which could contribute to tumorigenicity. Raw analytical data must be processed with a robust bioinformatics workflow to identify potential clonal expansions as well as annotate the breakpoint/integration site based on proximity to known oncogenes. Download the technical note below to compare ISA data from industry standard methods and find the best path forward for your lentiviral-based therapy.
FEATURES & Benefits
Integration Site Analysis Highlights
| Sample Type | Analytically Validated Methodologies | Estimated Completion Time | Data Deliverables* | Adjacent Services | |
| Peripheral blood mononuclear cells (PBMC), cell pellet, whole blood, tissue, gDNA transduced sample |
– Target enrichment sequencing / hybridization – capture (TES) – Quantitative shearing linear amplification mediated – PCR (qsLAM) |
Approximately 4 weeks |
– Integrations and frequencies – Clone characterization – Oncogene analysis – Integration hotspots – Longitudinal profiling – Vector integrity (TES) |
– AAV ISA – Vector Copy Number (digital PCR VCN analysis) – Whole Genome Sequencing (WGS) – On & off target analysis |
|
*A comprehensive report containing the data deliverables listed above is provided after ISA completion within our clinical laboratory.