AAV Vector Production (AAV Plasmid Synthesis)
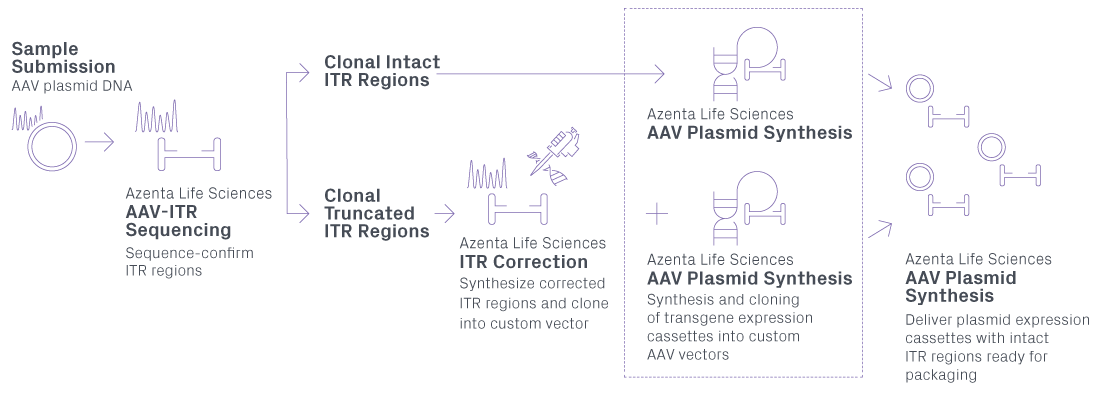
AAV Vector Production service provides the synthesis and cloning of transgene expression cassettes into custom AAV vectors with high efficiencies. Utilizing our decade-plus experience in synthesizing DNA, GENEWIZ from Azenta Life Sciences has developed a unique adeno-associated virus vector production service, the first of its kind, for researchers working with adeno-associated virus vectors. GENEWIZ AAV Vector Production service, listed as AAV Plasmid Synthesis in our CLIMS4 ordering system, comes bundled with our AAV Plasmid Preparation protocol to deliver mini- to giga-scale AAV plasmids that have been sequence-verified by our AAV-ITR Sanger Sequencing service.
We understand the importance of intact inverted terminal repeat (ITR) regions in downstream AAV gene therapy applications. GENEWIZ maintains a 2-step QC process and ITR correction by gene synthesis to ensure the integrity of your ITR regions.
PROPRIETARY AAV PLASMID SYNTHESIS WORKFLOW

AAV-ANCED STRATEGIES
For Adeno-Associated Virus in Gene Therapy
✓ Reliable quality control and safety testing for AAV development
✓ Strategies to read through inverted terminal repeats in AAV
✓ High-fidelity methods for AAV plasmid prep and packaging
✓ Advanced sequencing for AAV quality and safety monitoring
AAV VECTOR PRODUCTION FEATURES & BENEFITS
-
Industry-Leading Turnaround TimeAnd competitive price to save your budget -
Ph.D.-level Technical SupportThroughout your project
GENEWIZ INVERTED TERMINAL REPEAT (ITR) CORRECTION
Original AAV inverted terminal repeat shows a deletion mutation. Regular PCR amplification cannot repair the sequence due to its repetitive nature. GENEWIZ can successfully deliver repaired inverted terminal repeat regions through specific ITR correction technique.

IMPORTANCE OF INTACT ITR REGIONS IN AAV GENE THERAPY RESEARCH
Each inverted terminal repeat region is important in downstream AAV gene therapy applications*
| Function | Effecting Region |
| Residue of Non-Recombinant AAV (r(AAV)) Encapsided DNA | A-A’ |
| Packing | A-A’ (trs), D |
| Productivity | B-B’, C-C’ |
| Encapsidation | A-A’, B-B’, C-C’, D |
| Affinity to Rep Protein | A-A’, B-B’, C-C’ |
| Transduction Efficiency | A-A’ |
| Replication | A-A’, D |
| Rescue | A-A’ (trs), D |
| Integration | A-A’ (trs), D |
* Savy, A., Dickx, Y., Nauwynck, L., Bonnin, D., Merten, O.W. and Galibert, L., 2017. Impact of inverted terminal repeat integrity on rAAV8 production using the baculovirus/Sf9 cells system. Human Gene Therapy Methods, 28(5), pp.277-289.
*Zhou, Q., Tian, W., Liu, C., Lian, Z., Dong, X. and Wu, X., 2017. Deletion of the B-B’and C-C’regions of inverted terminal repeats reduces rAAV productivity but increases transgene expression. Scientific reports, 7(1), pp.1-13.
*Maurer, A.C. and Weitzman, M.D., 2020. Adeno-Associated Virus Genome Interactions Important for Vector Production and Transduction. Human Gene Therapy, 31(9-10), pp.499-511.
*trs – terminal resolution site

Tech Note | Optimizing Plasmid Preparation to Increase Yield and Reduce Endotoxins
Plasmid DNA (pDNA) is vital for a range of biopharmaceutical and biotechnological applications but maximizing yield while minimizing endotoxins can be challenging. This tech note explores strategies to optimize bacterial growth conditions, enhance lysis, and refine purification protocols to boost pDNA yield and purity, ensuring consistent, high-quality results.

Tech Note: An Efficient and Reliable Approach to AAV Packaging
While AAV is a powerful vehicle for gene delivery, unstable ITRs threaten the fidelity of rAAV genomes. Loss of ITR integrity diminishes the packaging efficiency of rAAV particles and thus viral titer. In this tech note, discover the effect of partial and full ITR deletions on AAV packaging and how to reliably identify and effectively correct them.

Tech Note: An Efficient and High-Fidelity Approach to AAV Plasmid Preparation
GENEWIZ has recently developed a proprietary AAV plasmid preparation process, which significantly improves the chance of maintaining ITR integrity, even when other commercially available competent cells growing in low temperature have failed. This process can also isolate clones with full-length ITR from a sample mixture containing intact and truncated ITRs.

Workshop & Roundtable Discussion: A Comprehensive Guide to Using AAV Vectors in Gene Therapy
Working with AAV vectors can be challenging. In this on-demand workshop & roundtable discussion, you’ll gain a better understanding of the AAV development pipeline for gene therapy research and learn how to optimize upstream and downstream processes including AAV synthesis, sequencing, bioinformatics, and storage.
Related Services
Learn how GENEWIZ can further support your AAV gene therapy research
