AAV-ITR Sequencing
GENEWIZ AAV-ITR Sequencing sequences difficult inverted terminal repeat (ITR) regions of adeno-associated virus (AAV), to expedite screening and validation of leads.
This newly-developed protocol prevents abrupt reduction in the sequencing signal at the start of the ITR hairpin and reads through the full length of the ITR region. AAV-ITR sequencing can be combined with our Primer Walking service to sequence whole AAV vectors.
Grants
Sanger Sequencing Grant:
Screen and confirm your results
Submit your application by March 31, 2026
Apply TodayGENEWIZ AAV-ITR Sequencing Provides a Breakthrough Solution
Adeno-associated virus (AAV) is one of the most actively investigated gene therapy vehicles. Our robust AAV-ITR Sequencing Protocol sequences through its difficult inverted terminal repeat (ITR) regions to better support your viral vector testing and drug discovery pipeline.
Standard Protocol
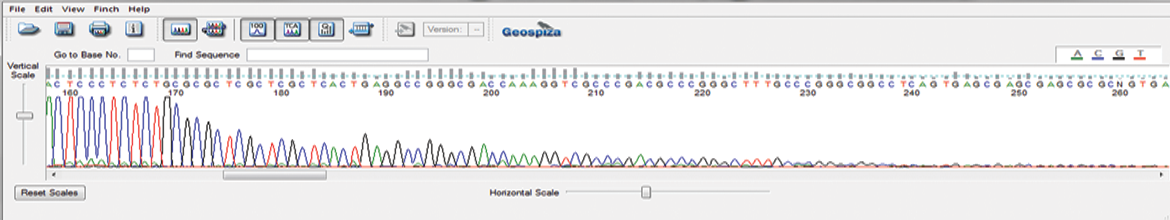
AAV-ITR Sequencing – GENEWIZ Proprietary Protocol
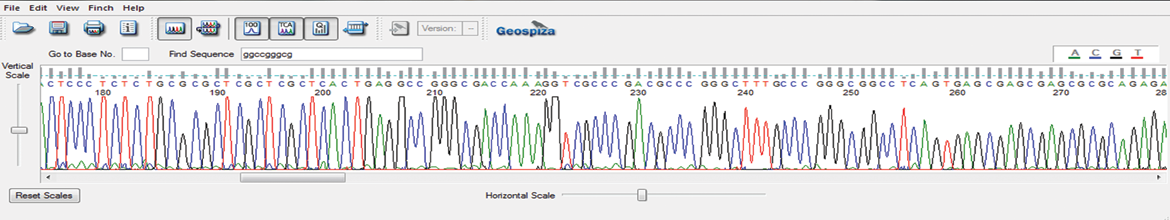
Features and Benefits
-
Qualitative assessment of the integrity of wild-type and partially-truncated ITR regions in AAV plasmids
-
Ph.D.-level customer support throughout the project
-
Turnaround time starting at just 5 business days
-
Increased read lengths and improved data quality for early detection of point mutations
-
Advanced network of dropboxes and couriers for convenient sample submission
-
Online ordering system allows real-time order management
Technical Resources
-

eBook | A Guide to AAV in Gene Therapy
AAV vectors play a critical role in gene therapy, offering an ideal approach for targeted gene delivery. However, understanding the complexities of AAV packaging, optimization, and quality control testing can be challenging. In this eBook learn step-by-step strategies for mastering AAV vector development and enhancing your gene therapy applications.
-

Tech Note | Reading Through the Inverted Terminal Repeats (ITRs) of Adeno-Associated Virus (AAV): GENEWIZ AAV-ITR Sanger Sequencing Method
GENEWIZ has developed a high-quality, direct Sanger sequencing method that reads through both intact and commonly mutated inverted terminal repeat (ITR) regions of adeno-associated virus (AAV). This method is an effective tool for assessing the integrity of ITRs in AAV plasmids.
-

Tech Note | An Efficient and Reliable Approach to AAV Packaging
While AAV is a powerful vehicle for gene delivery, unstable ITRs threaten the fidelity of rAAV genomes. Loss of ITR integrity diminishes the packaging efficiency of rAAV particles and thus viral titer. In this tech note, discover the effect of partial and full ITR deletions on AAV packaging and how to reliably identify and effectively correct them.
-

Blog | Optimizing AAV Plasmid Preparation and ITR Sequencing
The adeno-associated virus (AAV) is a powerful vehicle for gene therapy; however, working with AAV vectors can be challenging. Spontaneous mutations in the inverted terminal repeat (ITR) regions can accumulate during plasmid propagation in bacteria. Find out how our scientists have optimized the AAV plasmid preparation process to deliver 100% accurate constructs.
