mRNA Synthesis
GENEWIZ mRNA synthesis services support mRNA research and therapeutic applications with industry-leading project completion time, a wide range of deliverables, and best-in-class quality.
With the development of in vitro transcription (IVT) methods, messenger ribonucleotides (mRNA) synthesis has become an emerging class of gene therapy. Serving as the molecular bridge between DNA and proteins, mRNAs and their in vitro transcription technology enable the concept of nucleic acid-encoded proteins for research and therapeutic purposes. The transient nature of mRNA activity also offers an alternative approach to gene editing and gene therapy for delivering genetic information into cells.
GENEWIZ Multiomics and Synthesis Solutions from Azenta Life Sciences equips researchers with a range of flexible and tailored solutions that save valuable time and accelerate innovation.
What are the steps of mRNA synthesis?
Messenger RNA (mRNA) synthesis begins with codon optimization of this sequence for optimal transcription. The gene is synthesized, cloned into a vector, and verified by Sanger sequencing. The plasmid DNA is then amplified, target mRNA sequence linearized, and used as a template for in vitro transcription.
Custom mRNA Synthesis Applications
How do you synthesize mRNA?
Synthetic mRNA comes from a template nucleotide sequence of DNA. The process starts with codon optimization of this sequence for optimal transcription. The gene is synthesized, cloned into a vector, and verified by Sanger sequencing. The plasmid DNA is then amplified, target mRNA sequence linearized, and used as a template for in vitro transcription.
mRNA TranScription Service

Featured Application: circRNA Therapeutic Development
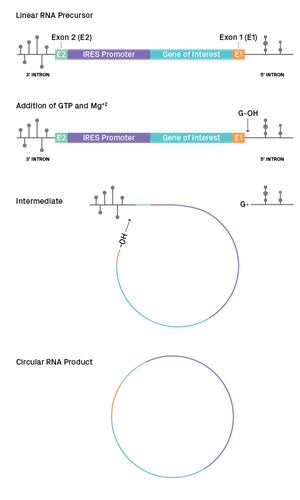
The circularization of coding RNAs into circRNAs has generated considerable interest as an approach to extend the duration of protein translation, thanks to their covalently closed structures and increased stability inside the cells. In the context of RNA-based therapeutics development, this has the potential to unveil better targets or gene delivery vectors with high pharmaceutical stability and biostability relative to current standard-of-care linear mRNA. GENEWIZ from Azenta has developed a proprietary and first-in-market workflow to produce high-quality circular RNA. Based on a group I intron self-splicing system, our process leads to improved circularization efficiency for high-yield circRNA products with favorable characteristics for therapeutic development.
mRNA Synthesis Service Features and Benefits
mRNA Synthesis Deliverables
mRNA and circRNA products are provided as dry powder, fully lyophilized and shipped at room temperature. Below is a summary of what to expect from your custom mRNA or circRNA synthesis order.
| mRNA Synthetic Motifs | Purified Synthetic Yield | Synthetic Length & Estimated IVT Completion Time | Quality Control |
| ncRNA: Non-coding/non-capped RNA | 100 ug 500 ug 1 mg 5 mg 10 mg Custom higher yields available |
< 4000 nt in as few as 1-2 weeks 4001-6000 nt in as few as 2-3 weeks > 6001nt, please inquire (custom project) |
– 100% sequence accuracy from Sanger sequencing of template – Certificate of analysis (CoA) – Gel electrophoresis size visualization – Agilent Bioanalyzer verification (optional)2 |
| Cap1 mRNA: m7GpppNm-capped mRNA with polyA tail | |||
| Cap0 mRNA: m7Gppp-capped mRNA with polyA tail | |||
| Pseudouridine modification (optional)1 | |||
| circRNA | 20 ug 50 ug 100 ug 500 ug 1 mg Custom higher yields available |
< 2000 nt in as few as 2-3 weeks 2001-4000 nt in as few as 3-4 weeks > 4001nt, please inquire (custom project) |
– 100% sequence accuracy from Sanger sequencing of template – RNaseR tolerance test – RT-PCR of circularization junction – Certificate of analysis (CoA) – Gel electrophoresis size visualization – Agilent Bioanalyzer verification (optional)2 |
Need mRNA fast for your pilot study? Below is a summary of what to expect from your off-the-shelf GFP mRNA order.
| coGFP3 control RNAs | Scale | Estimated Completion Time |
| Linear with 5’ cap 1 and a ~100A tail | 100 ug | As fast as 1 week |
| Circular RNA | 20 ug | In as few as 2 weeks |
1. Pseudouridine is a common RNA modification to replace uridine in IVT mRNA. It has been demonstrated to enhance RNA stability and decrease anti-RNA immune response.
2. Agilent Bioanalyzer utilizes LabChip technology to automate the measurement of both the concentration and the integrity/purity of mRNA samples.
3. Green fluorescent protein (GFP) 2 from Pontellina plumata, also known as ppluGFP2 (Shagin et al., 2004).
If you have custom requests or require any pre-project consultation, please contact our expert project management team.
Custom mRNA Synthesis Technical Resources

Tech Note | High-Fidelity Production of In Vitro Transcription Plasmids with Long Poly(A) Sequences
Poly(A) tail sequences can impact the integrity of your mRNA plasmids for in vitro transcription. While longer poly(A) tails have been shown to increase their stability, tails greater than 100 bases are susceptible to truncations. Learn how the GENEWIZ proprietary mRNA plasmid preparation protocol can help you generate higher yields and preserve poly(A) tails of greater lengths compared to standard protocols for high-fidelity templates in our tech note.

Tech Note | mRNA Production via Integrated Gene Synthesis and In Vitro Transcription
In vitro transcription (IVT) is a powerful technique to generate messenger RNA (mRNA) for a wide array of research and therapeutic applications. Transfection of mRNA enables very high protein expression, comparable to viral systems, with rapid onset. Read our tech note to learn how IVT, coupled with gene synthesis, can be used to synthesize mRNA of virtually any sequence quickly and efficiently.

Poster | Optimizing mRNA Vaccines and Virus-Mediated CAR T-Cell Therapy for Immuno-Oncology
Lentiviral vectors have proven effective at delivery of genetic material or gene editing technology for ex vivo processing, but the benefits and promise of Adeno-associated virus (AAV) and mRNA tools for in vivo immunotherapy have garnered recent interest. In this poster, we present streamlined approaches for lentiviral, AAV, and mRNA production that improves data quality through innovative synthesis and sequencing methodologies as compared to current standards






